The path to recovering from significant muscle trauma has long been fraught with challenges, often requiring multiple surgeries and leaving patients vulnerable to infection or long-term complications. The development of self-powered biodegradable implants represents a significant advancement in regenerative medicine and biomedical engineering. This review will explore the evolution of this technology, its key features, performance metrics, and the impact it is poised to have on various clinical applications. The purpose of this review is to provide a thorough understanding of the technology, its current capabilities, and its potential for future development.
An Introduction to Transient Bio-Electronics
The core principle of a self-powered biodegradable implant is elegantly simple: a medical device that actively promotes healing and then safely dissolves into the body once its job is done. These closed-loop systems represent the frontier of transient bio-electronics, a field dedicated to creating smart medical devices that perform a function and then disappear without a trace. This approach eliminates the need for secondary removal surgeries, which carry their own risks of infection and mechanical failure, offering a safer and more efficient path to recovery, particularly for deep-tissue injuries where permanent implants are impractical.
This technology has emerged as a direct response to the limitations of traditional medical devices. By integrating power generation, therapeutic delivery, and biodegradability into a single platform, these implants offer a holistic solution. The muscle defect-electrical stimulation (MD-ES) system is a leading example, combining energy harvesting with regenerative scaffolds to create a fully autonomous healing environment within the patient’s body. Its relevance extends beyond muscle repair, setting a precedent for how future medical implants across various disciplines could be designed for temporary, targeted interventions.
Core System Architecture and Functional Principles
Kinetic Energy Harvesting via Piezoelectric Film
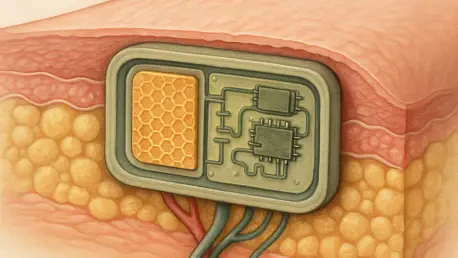
At the heart of the implant’s self-sufficiency is a piezoelectric film designed to harvest kinetic energy. Placed strategically near a joint or area of frequent movement, this component converts the body’s natural motions—walking, bending, or even breathing—into electrical energy. This process is fundamental to the implant’s operation, as it provides a continuous and reliable power source without the need for cumbersome external batteries or invasive wiring.
The film consistently generates a stable 500-millivolt electrical signal, which is sufficient to power the therapeutic components of the device. The significance of this battery-free design cannot be overstated. It not only reduces the implant’s physical footprint but also removes the risks associated with battery degradation, leakage, and the eventual need for surgical replacement, making the entire system truly transient and self-contained.
Conductive Hydrogel Scaffold for Tissue Regeneration
The therapeutic action of the implant is delivered by a conductive hydrogel scaffold positioned directly at the injury site. This advanced biomaterial serves a critical dual purpose. Firstly, it acts as an electrode, channeling the harvested electrical energy to the damaged tissue. This sustained electrical stimulation has been shown to significantly promote the growth and proliferation of new muscle cells, accelerating the natural healing process.
Secondly, the hydrogel provides a physical framework, or scaffold, upon which new tissue can grow and integrate. Its structure mimics the body’s own extracellular matrix, encouraging cells to attach and form organized muscle fibers. As the new tissue matures, the hydrogel scaffold gradually and safely biodegrades, leaving behind fully restored, functional muscle without any foreign material remaining in the body.
Recent Innovations and Preclinical Success
Recent breakthroughs have propelled this technology from concept to proven application, highlighted by the success of the MD-ES system in rigorous preclinical trials. In studies involving rats with severe muscle defects, the implant demonstrated remarkable efficacy. The combination of self-powered electrical stimulation and a regenerative scaffold led to complete muscle recovery in just 14 days—a timeline far exceeding conventional treatment outcomes.
Moreover, these trials confirmed the implant’s safety and transient nature. The entire device was fully and safely resorbed by the body within four weeks, leaving no harmful residues. This successful demonstration of both function and dissolution marks a critical milestone. It validates the integrated design and proves that transient bio-electronics can offer a powerful, automated recovery pathway, paving the way for upcoming human trials.
Potential Clinical Applications and Impact
The real-world implications of this technology are vast, with the potential to revolutionize the treatment of severe muscle injuries. The most immediate applications are in sports medicine, where athletes require rapid and complete recovery to return to peak performance. The automated, self-regulating nature of the implant could drastically shorten rehabilitation periods and reduce the likelihood of re-injury by ensuring a more thorough and structurally sound healing process.
Beyond athletics, the technology offers profound hope for elderly patients suffering from sarcopenia or significant muscle loss due to injury or surgery. For this demographic, invasive procedures and long recovery times pose significant risks. A biodegradable, self-powered implant could provide a minimally invasive solution to restore muscle mass and function, dramatically improving mobility, independence, and overall quality of life without the burden of a permanent device.
Challenges and Future Considerations
Despite its promising preclinical results, the transition of this technology to widespread human use faces several challenges. Scaling the device for human anatomy presents a significant technical hurdle; an implant designed for a rat must be re-engineered to accommodate the larger forces and tissue volumes in humans while maintaining its power efficiency and biodegradable properties. Ensuring consistent performance across a diverse patient population will require extensive optimization.
Furthermore, the regulatory landscape for novel bio-electronics is complex. As a first-of-its-kind transient device, it will require a rigorous approval pathway to establish long-term safety and efficacy standards. Market adoption will also depend on demonstrating cost-effectiveness compared to existing treatments. Ongoing development is focused on addressing these limitations by refining materials and manufacturing processes to ensure the technology is not only effective but also scalable and accessible.
Future Trajectory of Smart Medical Implants
The trajectory for this technology points toward increasingly sophisticated and autonomous medical implants. Future developments will likely focus on enhancing material durability and power efficiency, allowing for longer-lasting therapeutic effects before resorption. Researchers are also exploring the adaptation of this platform for other tissue types, such as bone, cartilage, and nerve tissue, which could open up new frontiers in regenerative medicine for a wide range of conditions.
In the long term, the evolution of self-powered biodegradable implants could lead to fully automated and personalized recovery paths. Future iterations might incorporate biosensors to monitor healing progress in real-time and adjust electrical stimulation accordingly, creating a truly smart system that adapts to the patient’s unique biological response. This would represent a paradigm shift from static treatments to dynamic, responsive therapies that actively manage the healing process from within.
Conclusion
The development of the self-powered biodegradable implant reviewed here constituted a pivotal step forward for transient bio-electronics. The successful integration of kinetic energy harvesting with a regenerative scaffold created a truly autonomous system for tissue repair. Its proven efficacy in preclinical trials and its inherent safety advantages over permanent implants established a new benchmark in regenerative medicine. This technology addressed critical limitations in treating severe muscle injuries and laid the groundwork for a new class of smart, absorbable medical devices that promised to redefine patient recovery.